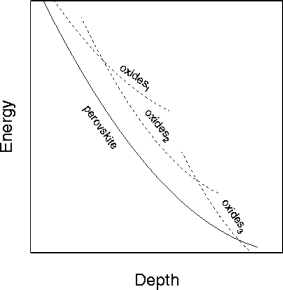
 Figure 1:
Schematic representation of energy versus depth for silicate
perovskite and various oxide assemblages. Disproportionation occurs
when an oxide assemblage attains a lower energy than the silicate
perovskite.
Figure 1:
Schematic representation of energy versus depth for silicate
perovskite and various oxide assemblages. Disproportionation occurs
when an oxide assemblage attains a lower energy than the silicate
perovskite.
Bina, C. R., and R. J. Hemley, Phase chemistry in the deep mantle, Abstracts of the Ocean Hemisphere Project (OHP) International Symposium on New Images of the Earth's Interior through Long-term Ocean-floor Observations, Kazusa Akademia Center, Chiba Prefecture, Japan, 109-110, 1997.
The top of the lower mantle is marked by the breakdown of spinel-structured (gamma) ringwoodite to a 1:1 mixture of ferromagnesian silicate perovskite (pv), (Mg,Fe)SiO3, and magnesiowüstite (mw), (Mg,Fe)O. At a similar depth, but distributed over a broader depth interval, the garnet (gt) components of the mantle also transform to silicate perovskite. (Mg,Fe)SiO3 perovskite is orthorhombic, while any calcium is taken up in a separate cubic CaSiO3 perovskite; aluminum is also soluble in the perovskite structure. This yields a simple first-order picture of lower mantle mineralogy: (Mg,Fe)SiO3 orthorhombic perovskite, (Mg,Fe)O magnesiowüstite, and CaSiO3 cubic perovskite, in decreasing order of abundance.
High pressure experiments and energetics calculations suggest that orthorhombic (Mg,Fe)SiO3 perovskite should remain stable throughout the lower mantle relative to to cubic or tetragonal distortions, and little compelling evidence of significant structural transition in orthorhombic ferromagnesian perovskite has yet been reported. (Mg,Fe)SiO3 perovskite solid solution exhibits a maximum solubility (varying with temperature and pressure) of FeSiO3 component. above which perovskite breaks down via a disproportionation reaction to mixed oxides: (Mg,Fe)SiO3 -> (Mg,Fe)O + SiO2. Reports of breakdown of Fe-free perovskite remain controversial. The perovskite structure is particularly susceptible to interesting ordering behavior, but Fe-bearing ordered silicate perovskites may not be quenchable to room temperature. Aluminum solubility in (Mg,Fe)SiO3 perovskite greatly enhances the solubility of ferric iron in this normally (ferrous) iron-averse phase, with resulting mixed-valence coordination further enhancing the scope for order-disorder behavior. Such Al-Fe3+ coupling complicates Mg-Fe-Al partitioning relations between gamma, gt, pv, and mw at the top of the lower mantle.
In cold subduction zones, pv does not appear promptly at ~660 km, but cubic garnet-majorite solid solutions remain stable. At greater depths, the gt phase is replaced by an ilmenite-structured phase (il) - either partially, with il+gt yielding to gt+pv (~700 km), or, in colder subduction zones, completely, with il consuming all gt and persisting until pv-only stability (~750 km). Moreover, ordered il also forms extensive solid solution with Al2O3. The degree to which il hosts ferric iron awaits further experimentation, but significant alumina solubility suggests a complex interaction between gamma, gt, il, and mw over a broad depth range.
Like orthorhombic ferromagnesian silicate perovskite, cubic CaSiO3 calcium silicate perovskite is subject to structural distortions, but, while unquenchable to room conditions, it has been found to persist over a range of pressures similar to that of the ferromagnesian phase. Recent calculations suggest, however, that CaSiO3, while generally believed to be cubic throughout the mantle, may actually possesses a weak orthorhombic distortion, suggesting possible transition to fully cubic form at higher pressures and temperatures. CaSiO3 perovskite may also dissolve significant alumina while undergoing orthorhombic distortion.
(Fe,Mg)O magnesiowüstite forms a complete solid solution in the NaCl or ``rocksalt'' structure, but the behavior of Fe diverges from that of Mg at high pressures. Fe-Mg partitioning relations between mw and pv vary significantly with pressure, temperature, and composition but exhibit increasing Fe partitioning into pv at high pressures. Solubility of Al in pv greatly increases Fe solubility (as Fe3+) in pv, decreasing ferric iron accommodation in mw at high pressure. Moreover, FeO undergoes a transition from rocksalt to a rhombohedral structure near 17 GPa, shifting to 25 GPa (~710 km) in (Fe0.9Mg0.1)O. Furthermore, FeO subsequently undergoes a rhombohedral to NiAs-type transition near 70 GPa (~1670 km), with the resulting inverse nickel arsenide structure suggesting progressive metallization of FeO. Finally, recent calculations suggest a possible ``magnetic collapse'' spin transition in FeO at core pressures, which MgO solid solution may shift to lower mantle pressures.
Rutile-structured stishovite undergoes a second-order transition to a CaCl2 structure near 50 GPa (~1250 km), followed by a a first-order transformation to an alpha-PbO2 structure near 80 GPa (~1870 km). Stishovite dissolves alumina to some extent, but the degree to which ferric iron may dissolve in stishovite, or either Al3+ or Fe3+ in the higher pressure silica phases, remains to be investigated. Alumina itself, in addition to its role in garnet, ilmenite, perovskite, and silica phases, transforms from Al2O3 corundum to an Rh2O3(II) structure near 90 GPa (~2070 km). Such phase transitions in MgO, FeO, SiO2, and Al2O3 may serve to destabilize pv relative to its component oxides, even when free oxide phases are not present in the mantle, by lowering the free energy of any mixed oxide assemblage (Figure 1).
Below 100 km depth, MgCO3 magnesite becomes the stable phase of carbonate throughout the mantle. Decreasing oxygen fugacity (fO2) with increasing pressure in the upper mantle reduces carbonate to diamond between 200 and 300 km, and the moderate solubility of Fe3+ in transition zone minerals preserves diamond stability, causing fO2 to continue to fall. A subsequent fO2 increase in the lower mantle was postulated, based upon strong partitioning of Fe into mw, but the recent discovery of high Fe3+ solubility in Al-bearing pv supports continued low fO2 values in the lower mantle and hence continued stability of the diamond phase of C. Furthermore, Fe3+-bearing pv allows electrical conductivity in the lower mantle to be controlled by the dominant pv phase rather than by accessory mw.
Water, on the other hand, possesses no primary host phases which are stable throughout much of the mantle. Hydrated magnesium silicates remain stable only in cold subduction environments, and any water in warmer mantle must reside in nominally anhydrous phases such as (beta) wadsleyite. In the lower mantle, the solubility of H in ferromagnesian pv is expected to increase with increasing Fe and Al substitution, and the solubility of H in stishovite also rises with increasing Al substitution. The possibly restricted nature of H2O transport and storage in the mantle is suggested both by the apparent absence of broadened seismic discontinuities in the transition zone and the absence of OH in olivine and orthopyroxene inclusions from diamonds.
In summary, entropy so dominates mineral thermodynamics at high temperatures that most phases form at least some degree of solid solution with others, lowering free energies and affecting transitions in real (as opposed to ideal end-member) compositions. Both structural distortion and solid solution involving charge-coupled substitution become common, and the attendant mixed-valence coordination and high pressures enhance order-disorder phenomena, especially among garnets and silicate perovskites. At high pressure, the behavior of Fe (a 3d64s2 transition metal) grows increasingly different from that of Mg (3s2). Both charge-coupled substitutions and Fe-Mg behavioral divergence are also seen in high pressure transitions in oxides, which may serve to destabilize silicate perovskite under certain conditions. Furthermore, it is through such substitutions that ferric iron enters lower mantle phases, allowing important reduction-oxidation reactions with volatile species such as CO2.
In terms of seismic structure, such phenomena may lead to complexity at the base of the transition zone, the top of the lower mantle, and the bottom of the lower mantle near the core-mantle boundary. Furthermore, changes in phase chemistry arising from local variations in pressure, temperature, or composition - e.g., those associated with slabs and plumes - may also generate complexity within the bulk of the lower mantle. Significant uncertainties remain, however. A general absence of experimental reversals means that the stability fields of many phases have not been clearly delineated. Similarly, many calculations and experiments have been performed at only a single temperature or composition, so that, in many cases, the dependence of transition pressure upon temperature (Clapeyron slopes) or composition (extent of solid solution) remains unknown. Finally, misleading quench effects - e.g., amorphization in CaSiO3 perovskite, anomalous elastic behavior and metastable back-transformation in (Mg,Fe)SiO3 pv, possible instability of Fe-rich ordered pv phases, generally non-recoverable extent of cation disorder - require in situ study of samples at high pressures and temperatures.
Acknowledgments. We are very grateful to the numerous researchers whose work is reviewed herein, many of whom kindly provided preprints of their work.
 Figure 1:
Schematic representation of energy versus depth for silicate
perovskite and various oxide assemblages. Disproportionation occurs
when an oxide assemblage attains a lower energy than the silicate
perovskite.
Figure 1:
Schematic representation of energy versus depth for silicate
perovskite and various oxide assemblages. Disproportionation occurs
when an oxide assemblage attains a lower energy than the silicate
perovskite.